Mastering Implantable Loop Recorder Coding: A Comprehensive Guide
** This article was reviewed and updated on January 27, 2024. **

The escalating demand for cardiovascular devices underscores the significance of implantable loop recorders (ILRs), especially in managing cardiac conditions arising from irregular heart rhythms and chronic diseases. With this surge in demand, medical coders must stay informed on new and revised codes and guidelines for accurately reporting these services.
Understanding Implantable Loop Recorders
Coding for ILRs requires a thorough understanding of the device’s purpose, how the procedure is performed, and the management of the device once it is inserted. The correct reporting of procedure codes hinges on adherence to the CPT coding guidelines specific to implantable loop recorders. Let’s delve into the intricacies of ILR coding and sharpen your skills with three related coding exercises.
Purpose of Loop Recorder Device
Referred to as subcutaneous cardiac rhythm monitors or insertable cardiac event recorders, ILRs are vital in monitoring the heart’s electrical activity over an extended period. According to Johns Hopkins Medicine, a physician may recommend ILRs for patients experiencing symptoms such as fainting (also known as syncope), palpitations, rapid or slow heartbeats, and hidden rhythms that may lead to strokes. The decision to implant an ILR is often made when conventional tests fail to determine the cause of these symptoms.
Differentiating ILRs From Portable Monitors
While ILRs and portable heart rhythm monitors, such as a Holter monitor, share the common goal of monitoring the electrocardiogram (ECG) for unexplained symptoms, they differ in application. ILRs are suitable for patients with less frequent symptoms, providing valuable insights to identify the root cause.
Short Video on an ILR Procedure
Below is a short video explaining an implantable loop recorder procedure.
Procedure for ILR Insertion
The insertion of an implantable loop recorder, a quick and straightforward procedure lasting under 15 minutes, is typically performed by an electrophysiologist as an outpatient procedure. According to the National Library of Medicine, lidocaine is injected for numbing into the left side of the body at about the second or third rib level over the heart. A small incision is made under the skin, and a pocket is created to insert the loop recorder, which is about the size of a USB memory stick and contains two self-contained electrodes. Once inserted, the device is activated, programmed for data collection, and the incision is closed.
Programming and Monitoring
Effective programming and continuous monitoring are imperative post-ILR insertion. The initial programming is performed during implantation, with subsequent adjustments as needed. Remote monitoring, facilitated by Wi-Fi or cellular connection, has become the standard of care, allowing for efficient data retrieval and review. Retrieved data must be reviewed at least once every 30 days.

Data is gathered from the implanted device as the patient undergoes regular daily activity. When there is an abnormal heart rhythm, the remote device automatically records it based on the algorithms and QRS signals. The patient may also activate the device at the time of the episode. These tracings are then transmitted to a receiving station, where a technician interprets the electrocardiographic (ECG) tracing, any reported symptoms, and the length of duration, which assists the physician in diagnosing the underlying problem.
ILR Removal
Removal of an ILR is recommended around the three-year mark when the batteries cease to record information. Earlier removal may be necessary if significant rhythm problems or cardiac events are detected and may be replaced with a pacemaker or implantable cardiac defibrillator. The removal procedure is similar to the insertion process, involving local anesthesia, a small incision, and closure.
If the cause of the symptoms is something other than a cardiac condition, an appropriate physician will treat it, and the patient may choose to leave the ILR device in place or remove it. Additional reasons a rhythm monitor may need to be removed include complications such as pain at the implant site, infection of the local pocket, or a local skin reaction to the device. Also, poor R-wave sensing, a rare complication, may require moving the device to another location.
CPT Coding for ILR Procedures
Insertion and Removal
In the CPT codebook, an implantable loop recorder is called a subcutaneous cardiac rhythm monitor (SCRM). SCRM codes are listed under Surgical Procedures on the Cardiovascular System, specifically under Surgical Procedures on the Heart and Pericardium.
These codes are classified as to whether the device is being inserted or removed:
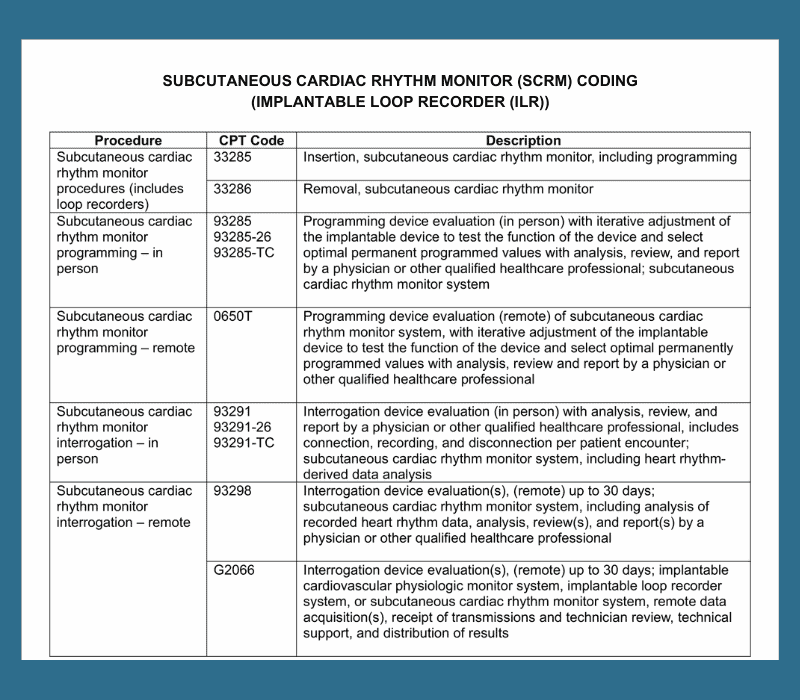
- 33285, Insertion, subcutaneous cardiac rhythm monitor, including programming
- 33286, Removal, subcutaneous cardiac rhythm monitor
The initial insertion includes programming. According to the CPT coding guidelines, subsequent electronic analysis and/or reprogramming should be reported with 93285, 93291, or 93298.
Programming and Interrogation
CPT Category I codes for programming (93285, 0650T) and interrogation (93291, 93298, G2066) devices are listed under Cardiovascular Procedures, specifically under Implantable, Insertable, and Wearable Cardiac Device Evaluations. The coding guidelines emphasize the need for modifiers in certain situations to ensure precise coding and billing practices.
Programming Codes
Programming of the subcutaneous cardiac rhythm monitor is reported according to whether the service is performed in person or remotely.
- 93285, Programming device evaluation (in person) with iterative adjustment of the implantable device to test the function of the device and select optimal permanent programmed values with analysis, review, and report by a physician or other qualified health care professional; subcutaneous cardiac rhythm monitor system
- 0650T, Programming device evaluation (remote) of subcutaneous cardiac rhythm monitor system, with iterative adjustment of the implantable device to test the function of the device and select optimal permanently programmed values with analysis, review, and report by a physician or other qualified health care professional
According to the CPT coding guidelines:
- 93285 cannot be reported with 33285, 93279-93284, 93291, or 0650T.
- 0650T cannot be reported in conjunction with 33285, 93260, 93279, 93280, 93281, 93282, 93284, 93285, or 93291.
Note: Codes 93260, 93279-93284 refer to the programming of pacemakers or defibrillators.
Code 93285 requires a modifier according to whether it is the professional or technical component of the service. For example, if only the professional component is performed, assign 93285-26; for only the technical component, assign 93285-TC. If both components are performed, report 93285 without a modifier.
A physician or other qualified healthcare professional must personally perform the professional component of the work to report the professional component-only codes.
The technical component-only code requires that the procedure be performed under the physician’s general supervision and control. The physician does not need to be personally present during the procedure.
Interrogation Codes
Interrogation codes are reported based on whether the monitoring is performed in person or remotely and whether the professional or technical component of the service is performed.
- 93291, Interrogation device evaluation (in person) with analysis, review, and report by a physician or other qualified health care professional, includes connection, recording, and disconnection per patient encounter; subcutaneous cardiac rhythm monitor system, including heart rhythm-derived data analysis
Code 93291 requires a modifier (-26, -TC) according to whether it is the professional or technical component of the service. If both components are performed, 93291 is reported without a modifier.
- 93298, Interrogation device evaluation(s), (remote) up to 30 days; subcutaneous cardiac rhythm monitor system, including analysis of recorded heart rhythm data, analysis, review(s), and report(s) by a physician or other qualified health care professional
- G2066, Interrogation device evaluation(s), (remote) up to 30 days; implantable cardiovascular physiologic monitor system, implantable loop recorder system, or subcutaneous cardiac rhythm monitor system, remote data acquisition(s), receipt of transmissions and technician review, technical support and distribution of results
Codes 93298 and G2066 do not need modifiers -26 or TC appended, as their code descriptors already indicate the technical or professional component.
According to the coding guidelines:
- Do not report 93291 in conjunction with 33285, 93288-93290, 93298, 0650T
- Do not report 93298 in conjunction with 33285, 93291, 93297, 99091, 99454
- Report 93298 only once per 30 days per patient and not less than 10 days
3 Coding Exercises: Test Your Skill
Assign the correct procedure code for each of the following and review the answers and rationales below.
#1. A 62-year-old female is seen by Dr. Blue, an electrophysiologist, for a new insertable cardiac event recorder after having unexplained syncope episodes and is suspected of cardiac arrhythmia. Dr. Blue anesthetized the patient’s chest area above the heart with injectable lidocaine and made a small incision in the skin. He then inserted and programmed the monitor before closing the area with two staples.
#2. A patient with an implanted loop recorder presents today to the cardiologist for the removal of the device after having it in place for two years. The patient states he has experienced no syncope episodes since the device was inserted, and the device detected no cardiac arrhythmias. The cardiologist numbs the chest area with injectable lidocaine and creates an incision. The device is removed, and the incision is sutured closed.
#3. A representative for the implantable loop recorder system manufacturer performs the technical remote monitoring of the device during a 30-day period. Under the physician’s supervision, the rep distributes the results to the physician’s office, and the physician analyzes the results and writes a report with the diagnosis of the underlying cause. How would the physician report for his portion of the service?
Answers and Rationales
#1. 33285
Rationale: One way to find the correct code in the CPT codebook is to go to the Index and look under Cardiac Event Recorder. The instructions tell us to see Subcutaneous Cardiac Rhythm Monitor System.” From there, it takes us to:
- Insertion 33285
- Interrogation Device Evaluation
- In Person 93291
- Remote 93298
- Programming Device Evaluation
- In Person 93285
- Remote 0650T
- Removal 33286
Since this is an insertion of a cardiac rhythm monitor, we need to go to 33285 in the Tabular to verify our correct code as:
33285, Insertion, subcutaneous cardiac rhythm monitor, including programming
The coding guidelines state that initial insertion includes programming. For subsequent electronic analysis and/or reprogramming, see 93285, 93291, 93298. Therefore, we should not assign a code for the programming of the device.
#2. 33286
Rationale: The device detected no arrhythmias, so the patient did not require a pacemaker or implantable cardioverter-defibrillator to correct the condition. Hence, the patient elected to have the device removed.
In the Index, if we look up Loop Recorder System, Implantable, it instructs us to see Subcutaneous Cardiac Rhythm Monitor System. Under this listing, it shows us Removal 33286. We can verify this code in the Tabular as:
33286, Removal, subcutaneous cardiac rhythm monitor
#3. 93298
Rationale: The physician performed only the professional review and interpretation of the remote interrogation device evaluation during a 30-day period. The manufacturer’s rep performed the technical portion of the monitoring. Therefore, the physician may only report for the professional component of the service.
In the Index, look up Monitoring/Cardiac/ Subcutaneous Cardiac Rhythm Monitor System/Interrogation device evaluation(s), (remote) up to 30 days, 93298. In the Tabular, we can verify this code as:
93298, Interrogation device evaluation(s), (remote) up to 30 days; subcutaneous cardiac rhythm monitor system, including analysis of recorded heart rhythm data, analysis, review(s), and report(s) by a physician or other qualified health care professional
CPT 93298 is to be reported once for each 30-day period on the 31st day. There is no need to append modifier -26 to 93298 as the code descriptor indicates it is the professional component of the service. A device industry representative under the direction of the physician who performed the technical component of the service would report G2066.
Codes that May Support SCRM Codes
According to Medtronic, a leading maker of medical devices and subcutaneous cardiac rhythm monitors, the following medical codes may support subcutaneous cardiac rhythm monitors:
ICD-10-CM codes:
G45.0-G45.3, G45.8-G45.9, Transient cerebral ischemia attacks and related syndromes
I47.0-I47.9, Paroxysmal tachycardia
I48.0-I48.19, Atrial fibrillation and flutter
I49.01-I49.9, Other cardiac arrhythmias
I63.0-I63.9, Cerebral infarction
I69.30-I69.998, Sequela of cerebral infarction
R00.2, Palpitations
R06.02, Shortness of breath
R07.89, Other chest pain (includes chest pressure, discomfort, and tightness)
R07.9 Chest pain, unspecified
R42, Dizziness and giddiness
R53.83, Other fatigue (includes lack of energy, tiredness
R55, Syncope and collapse (pre-syncope)
R94.31, Abnormal electrocardiogram (ECG) (EKG)
Z79.01, Long-term (current) use of anticoagulants
Z86.73 Personal history of transient ischemic attack (TIA), and cerebral infarction without residual deficits
HCPCS code:
C1764, Event recorder, cardiac (implantable)
The above is not an all-inclusive list.
Conclusion
Mastering implantable loop recorder coding involves a comprehensive understanding of the device, procedures, and coding guidelines. This guide aims to equip medical coders with the knowledge required to navigate this evolving landscape, ensuring accurate reporting and reimbursement for these essential cardiovascular services.
